Background
In response to rising concerns about the integrity of their supply chain, one of our mid-sized pharmaceutical clients recently undertook a project to combat the proliferation of counterfeit drugs within the Asia-Pacific (APAC) region. Noticing unusual patterns and inconsistencies, they sought to gauge the extent and impact of fraudulent pharmaceuticals circulating in these markets.
Solution
Utilizing the sophisticated capabilities of the TrueMed Digital Lab system, the company initiated a rigorous detection and analysis process to identify and address the counterfeit drug issue.
At A Glance
Challenges
- Observing Irregular product variations
- Reports of counterfeits
- Unclear extent of problem
Findings
High-risk countries Identified: Indonesia, Singapore, Thailand & Philippines –
73% of the sellers were offering fakes unknowingly
Findings
The in-house investigator team completed 242 test purchases across South-East Asia, with 207 confirmed through the TrueMed app. Analysis of the 207 samples revealed 169 (82%) to be counterfeit, and contained six counterfeit variants among them.
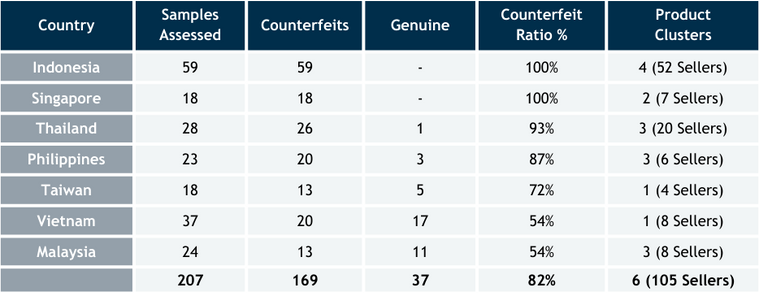
- High-risk countries: Indonesia, Singapore, Thailand & Philippines – 73% of the sellers offering fakes
- Shipping locations:112 products were shipped locally;18 products were shipped cross border & 77 locations not disclosed


